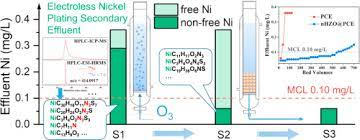
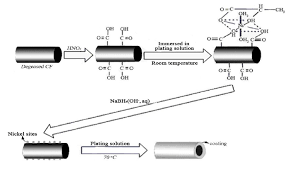
Electroless nickel plating, like that from Poeton is essentially a two-step chemical procedure which deposits an evenly neutralized layer of nickel phosphate on the surface of an inert metal, such as steel or aluminum. The active ingredient used in this process is a mixture of alkaline chemicals and a nitrous oxide. When the two chemicals react with each other, they form a positive charge. This is similar to the results of electroplating, where a direct current (DC) is used to pass through a positively charged plate, causing the plate to create a phosphorous bond between the two chemicals. The main difference is that in electroplating, there is an extra step before the positive charge is created. Nickel plating, however, does not require any extra step to produce a highly effective electrical conductive layer.
Another major advantage of electroless nickel plating over other forms of plating is that it does not require any heavy equipment to carry out the process. For starters, the deposited metal can be simply rubbed over a non-metallic base, such as copper, which will cause a good level of corrosion resistance. In addition, the coating can also be applied directly to a working piece, avoiding the need for further welding or hot work. These plating solutions also offer the ability to apply the coating with a brush or paint roller, eliminating the use of specialized equipment such as die stamps or a tig torch.
There are some limitations to electroless nickel plating. First, the efficiency of the plating solution may be lower than that of hot-worked deposits. Also, some manufactures prefer the use of hot-worked plating solutions because these solutions are available in higher temperatures. Lastly, these plating solutions do have a relatively short lifespan, typically being effective for a lifespan of only about two to three years. The manufacturers of these deposits also recommend the regular replacement of electrolytes and the re-varnishing and waxing of deposits made from nickel, as these products contribute to the aging of the metal.